UNUB At Boyle temperature, the value of compressi factor Z has a

Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

Course Outline: Particulate Nature of Matter, PDF, Gases
Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora

and two-phase flow in singular geometries and safety relief valves

Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect

Solved The plot below shows how compressibility factor (Z)

EGR 334 Thermodynamics Chapter 3: Section ppt video online download

Respostas - Físico-Química (Vol.1) - Atkins PDF
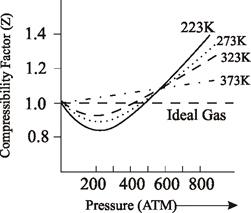
Chemistry Desk: Effect of Temperature
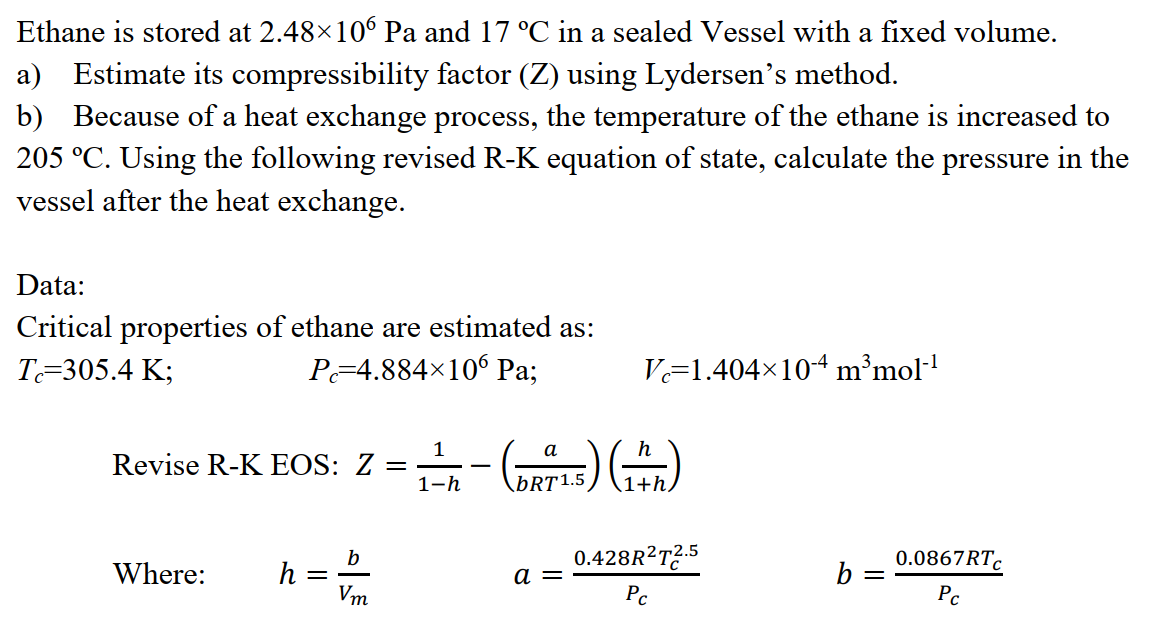
Solved Ethane is stored at 2.48x10° Pa and 17 °C in a sealed

Real Gas Behavior The Compression Factor (Z) [Example #2]

Solved (b) The compressibility factor Z of a non-ideal gas

PPT - GASES PowerPoint Presentation, free download - ID:2088317

C11 HSC Chemistry Text Book PDF, PDF

and two-phase flow in singular geometries and safety relief valves

Deviation From Ideal Gas Behavior - Study Material for IIT JEE