SOLVED: A 35-g ice cube at 0.0 °C is added to 110 g of water in a 62-g aluminum cup. The cup and the water have an initial temperature of 23 °C. (
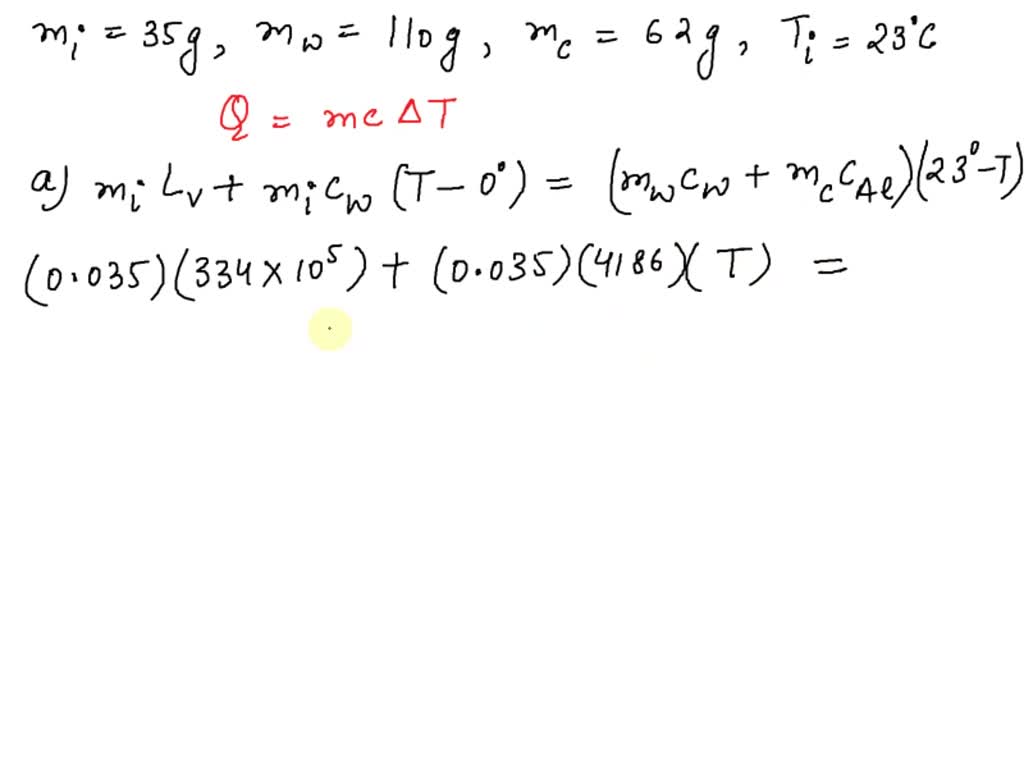
VIDEO ANSWER: Hello students to solve the given question: let us use the equation of heat transfer that is equal to m c c. Here is the specific heat capacity multiplied by delta t that is, temperature difference now, using this relation? Let us solve
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
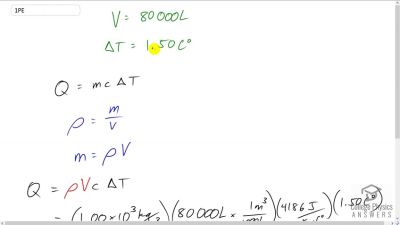
Chapter 14: Heat and Heat Transfer Methods
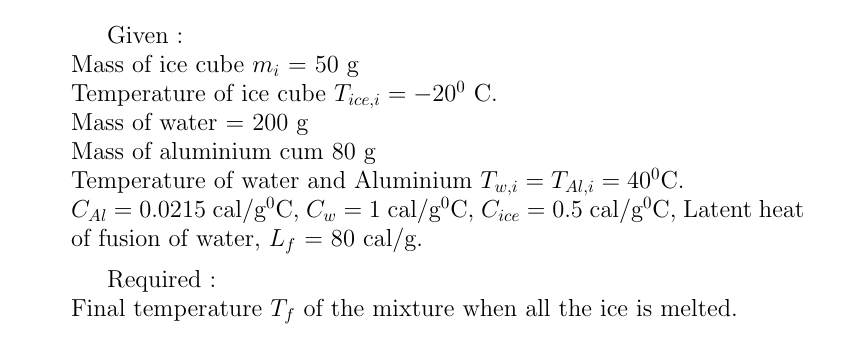
Answered: A 50 g ice cube, initially at -20degree…
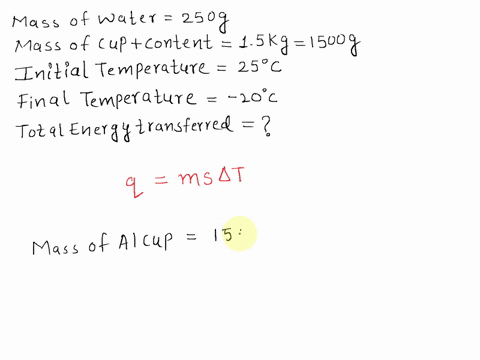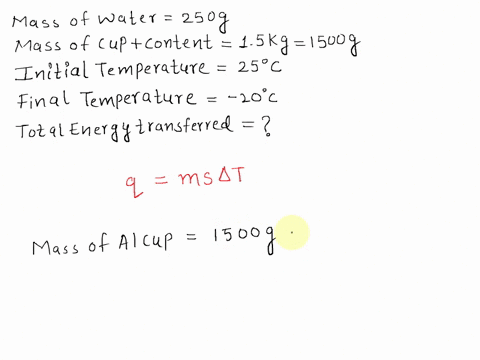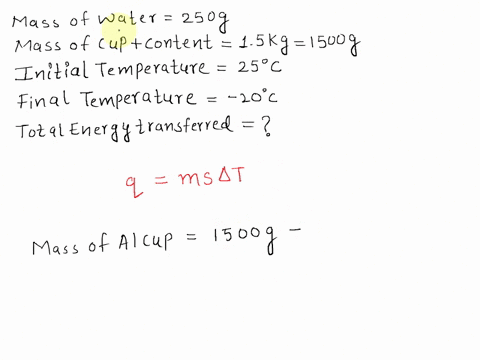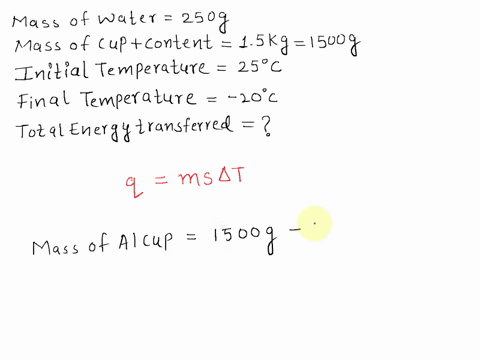
SOLVED: A 15-g ice cube at 0 °C is placed in an aluminum cup

⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added
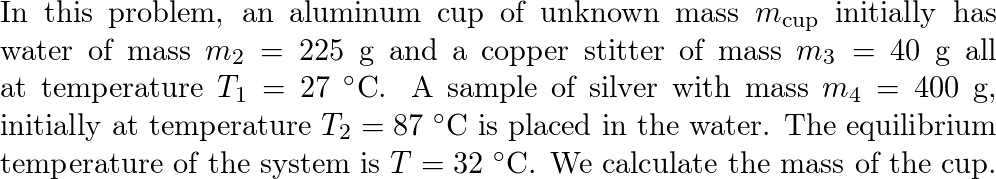
An aluminum cup contains 225 g of water and a 40-g copper st
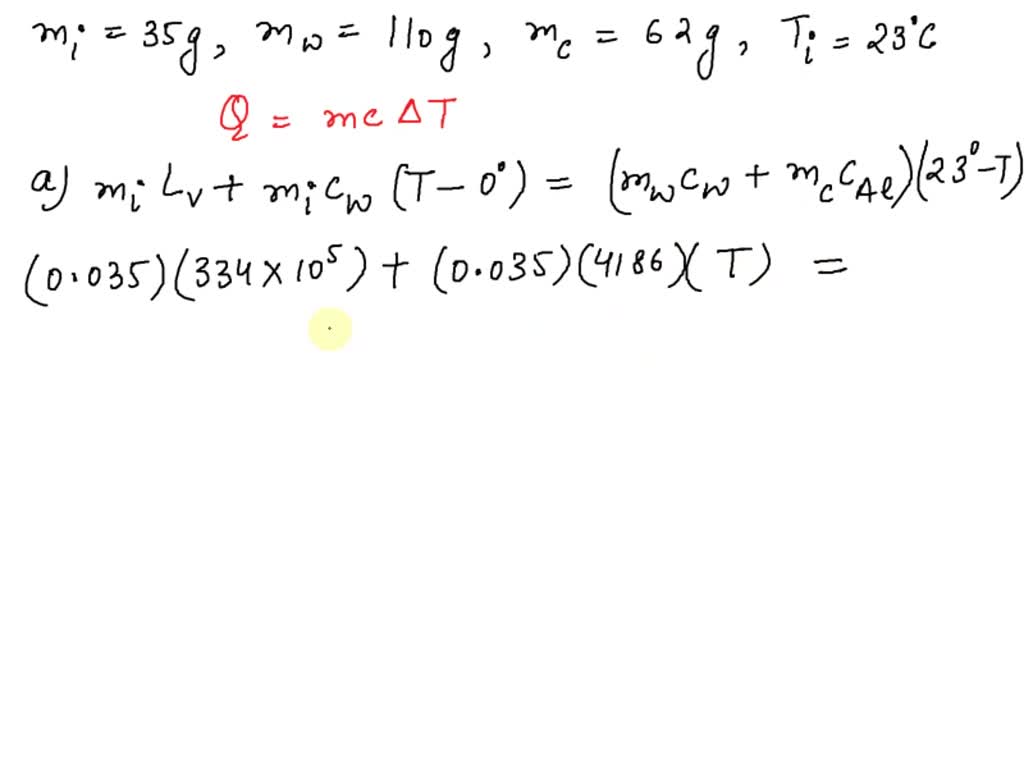
SOLVED: A 35-g ice cube at 0.0 °C is added to 110 g of water in a

Specific Heat Capacity

Steam at 100°C is added to ice at 0°C. (a) Find the amount of ice
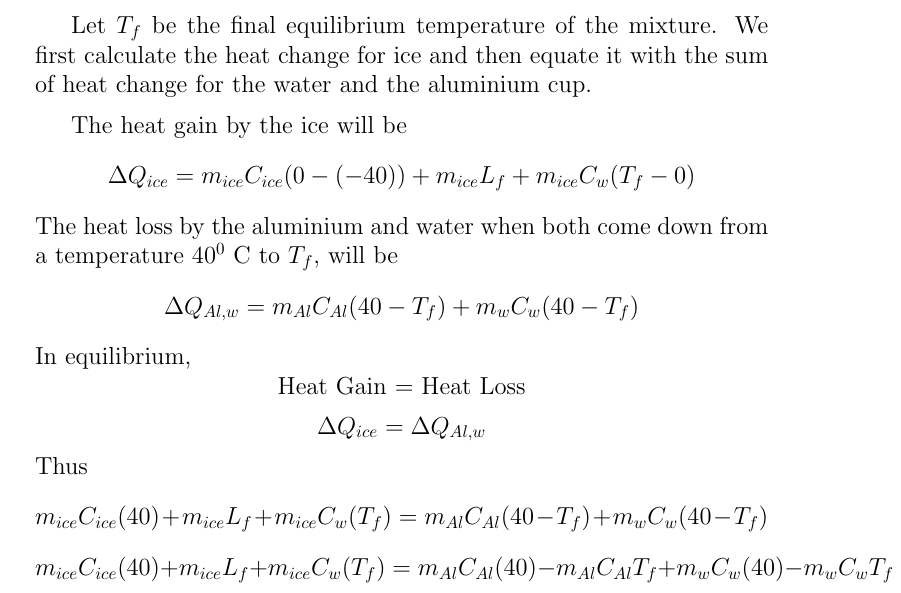
Answered: A 50 g ice cube, initially at -20degree…

Equilibrium Temperature of water in cup