32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
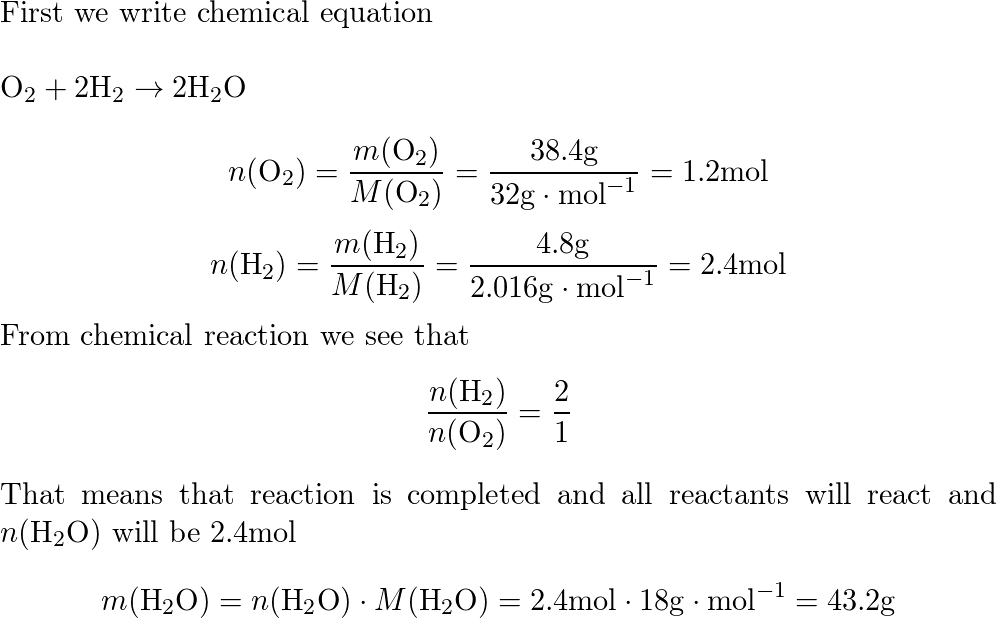
Hydrogen and oxygen react chemically to form water. How much

80 g of `H_(2)` is reacted with 80 g of `O_(2)` to form water

CO2 Decomposition in CO2 and CO2/H2 Spark‐like Plasma Discharges

ugures. i 64 of H, reacts with 32 g of Oz to yield water. Which is the limiting reactant? mass of water produced and the amount of excess reagent left. i) Explain
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?

Chapter 3 Chemical Reactions and Reaction Stoichiometry - ppt download
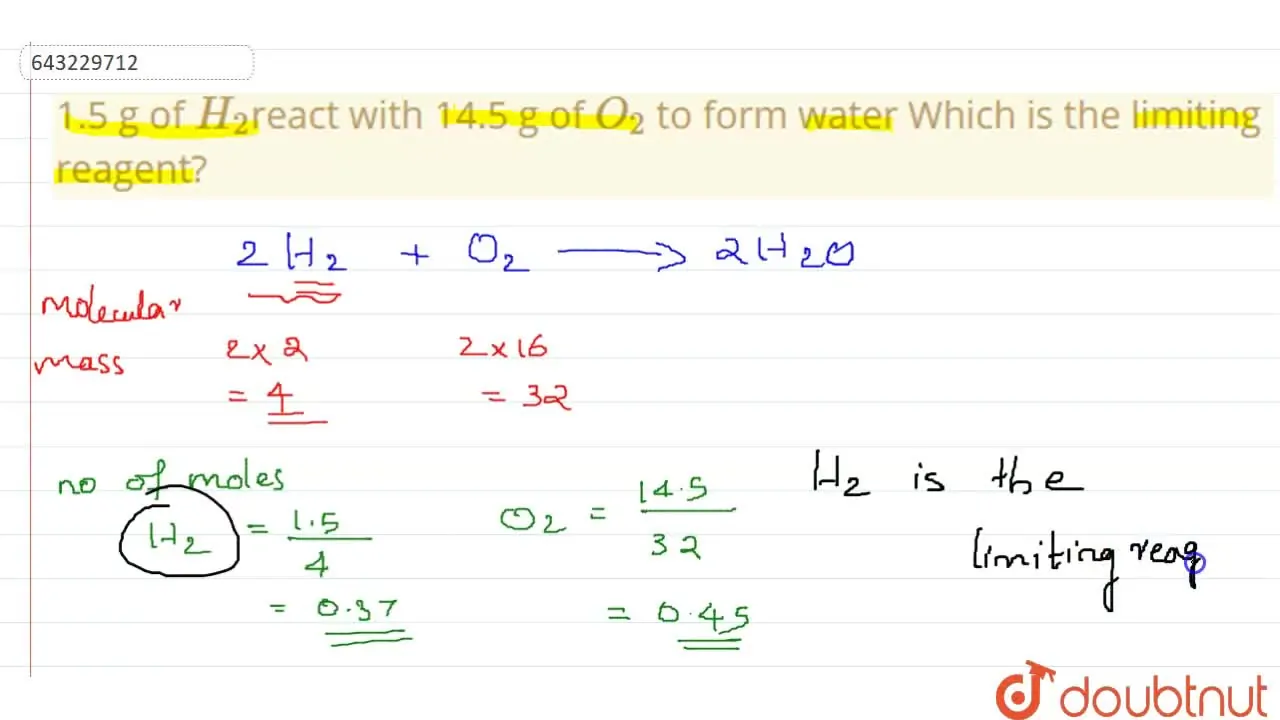
Malayalam] 1.5 g of H2react with 14.5 g of O2 to form water Which is

Recent Advances in Electrochemical Water Oxidation to Produce

80g of H2 is reacted with 80g of O2 to form water; what are the

SOLVED: 3.0 g of H2 react with 29.0 g of O2 to form H2O (i) which