Compressibility factor (Z) for a van der Waals real gas at

Share your videos with friends, family and the world

Compressibility factor (gases) - Citizendium

Compressibility Factor Z Important Concepts and Tips for JEE Main

Description of real gases: Compression factor
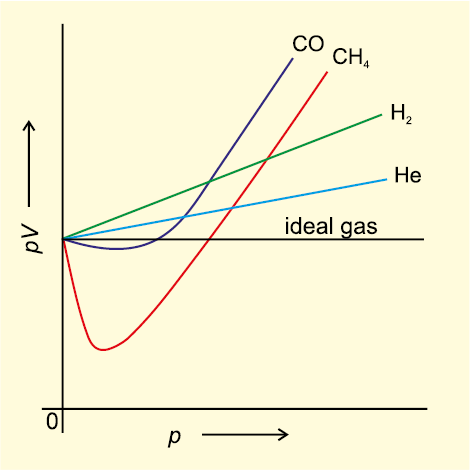
Sections
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
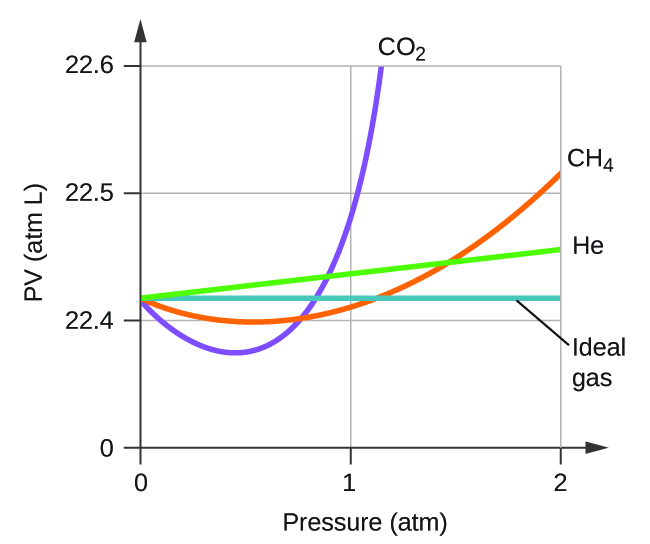
Non-Ideal Gas Behavior – Chemistry
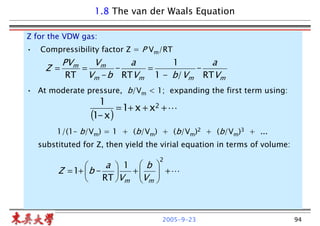
Real Gases and the Virial Equation
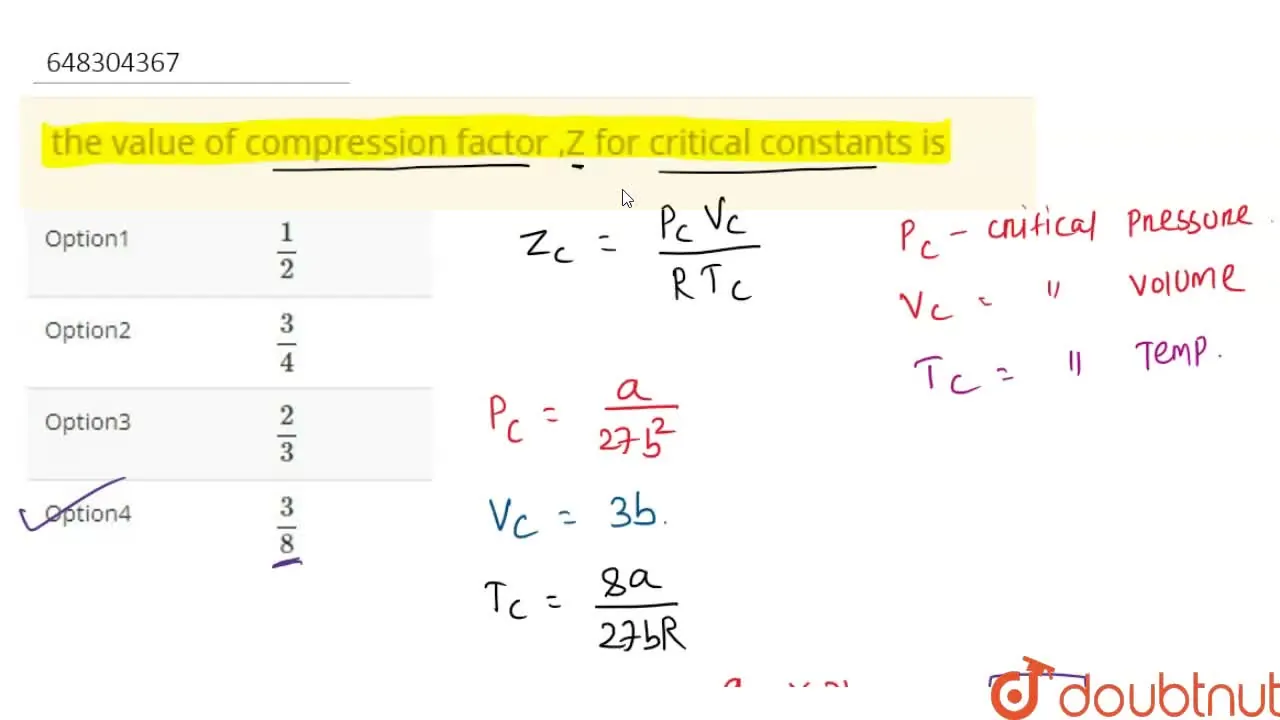
the value of compression factor ,Z for critical constants is
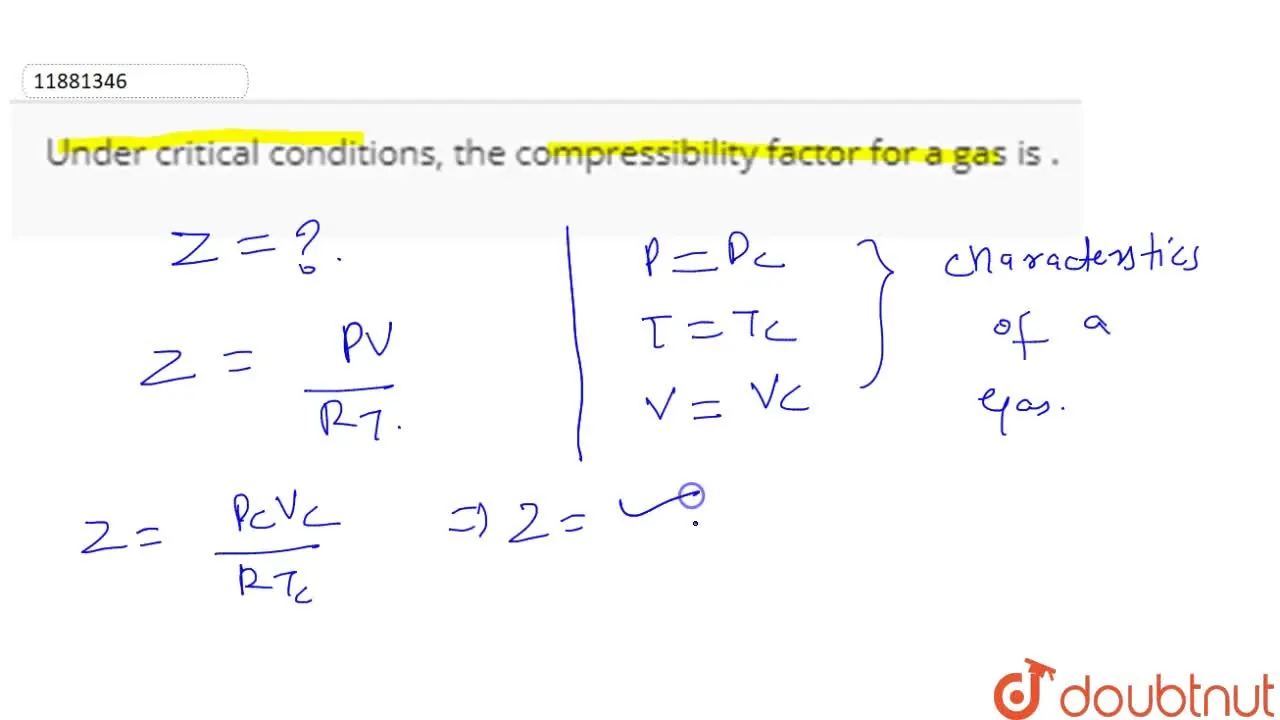
Under critical conditions, the compressibility factor for a gas is .
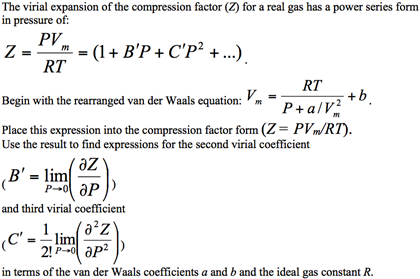
Solved The virial expansion of the compression factor (Z)
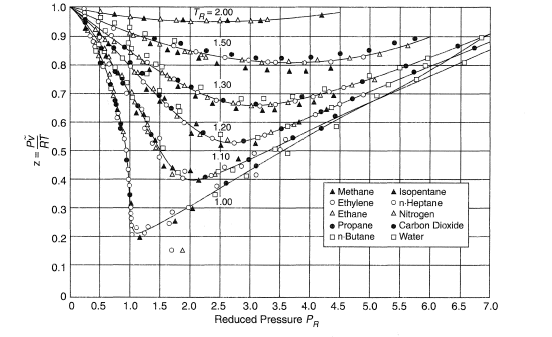
GAS LAW

Compressibility of a van der Waals Gas, Physical Chemistry I

The, compressibility factor (Z) of one mole of a van der waals gas of negligible a value is: a.

Derivation of Van Der Waals Equation