Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

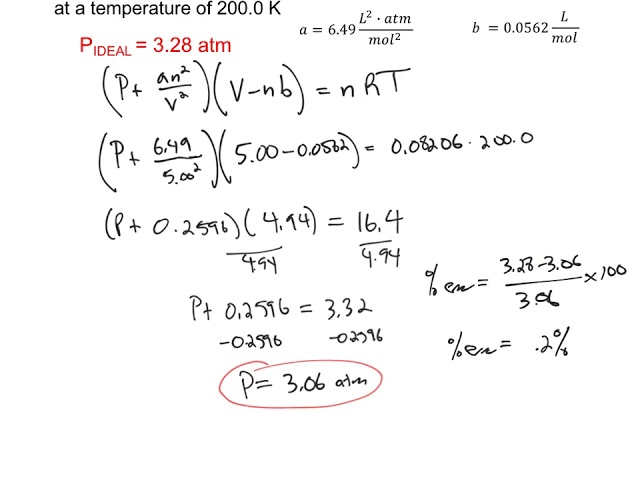
van der Waals example

The compression factor (compressibility factor) for 1 mol of a van der
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

The compression factor (compressibility factor) one mole of a van der Waals'gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible

List references from the University of Geneva Physical Chemistry reference database

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

1148 questions with answers in GAS

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

Solved APPENDIX Problem 1: Molar Volume and Compressibility
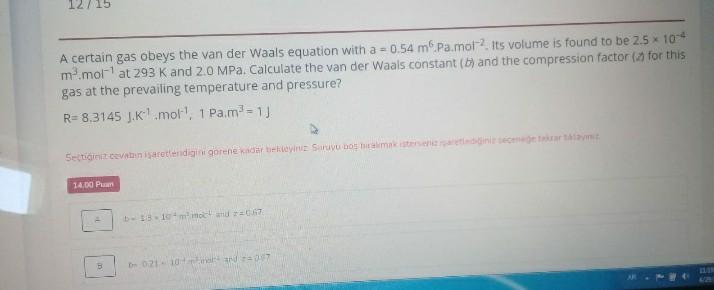
Solved A certain gas obeys the van der Waals equation with

1148 questions with answers in GAS