If Z is a compressibility factor, van der Waals equation at low

Solution For If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 1: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 2: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 3: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 4: If Z is a compressibility factor, van der Waals equation at low pressure can be written as

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
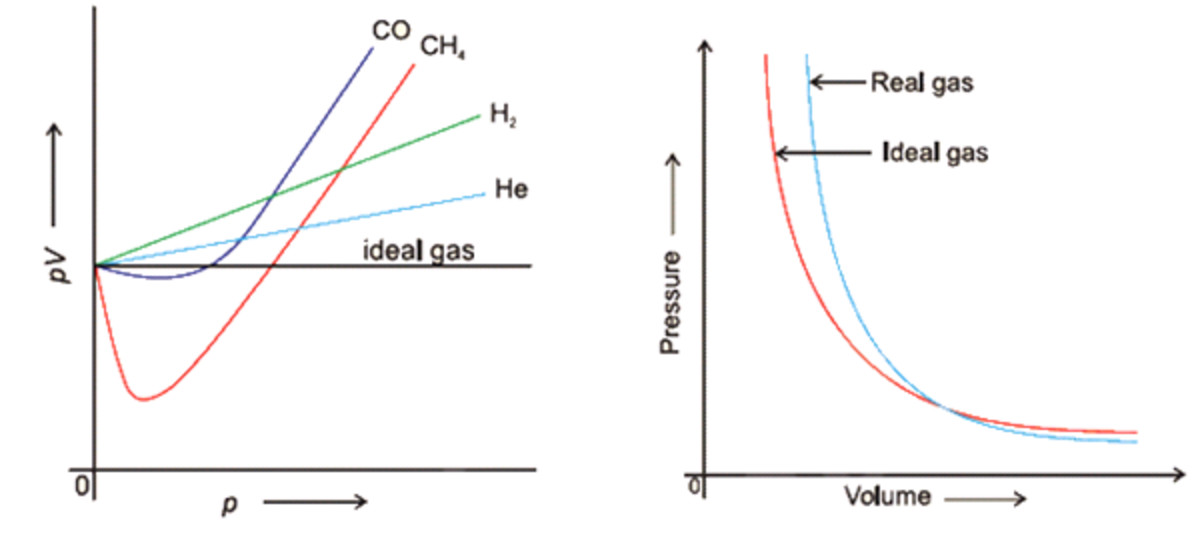
Real Gases - Chemistry, Class 11, States of Matter

At low pressures, the van der Waals equation is written as [P+(a)/(V^(

⏩SOLVED:If Z is a compressibility factor, van der Waals equation

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Van der Waals equation - Wikipedia

Explain how the compression factor varies with pressure and

If Z is a compressibility factor, van der Waals' equation at low press

SOLVED: I need the answer as soon as possible. 16. If Z is a compressibility factor, van der Waals' equation at low pressure can be written as: (P + (an^2/V^2))(V - nb) =

Critical Constants and the Van Der Waals Equation of State
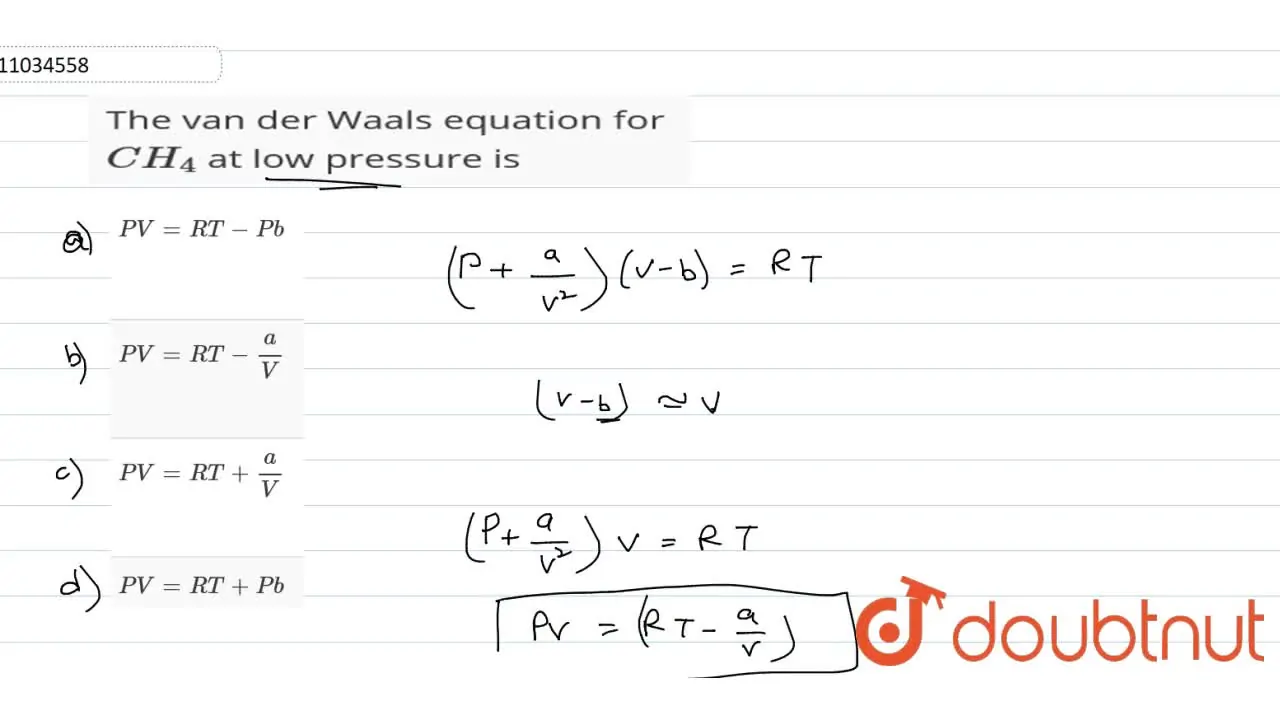
The van der Waals equation for CH(4) at low pressure is

If Z is a compressibility factor, van der Waals equation at low pressure ..