What is the change in internal energy (in J) of a system that absorbs 0.464 kJ of heat from its surroundings and has 0.630 kcal of work done on it?

I found an increase of 3100J Have a look

HVAC Engineer's Handbook, Eleventh Edition [11 ed

ME532 AdvancedHT IIConvectionandMassTransfer PDF, PDF
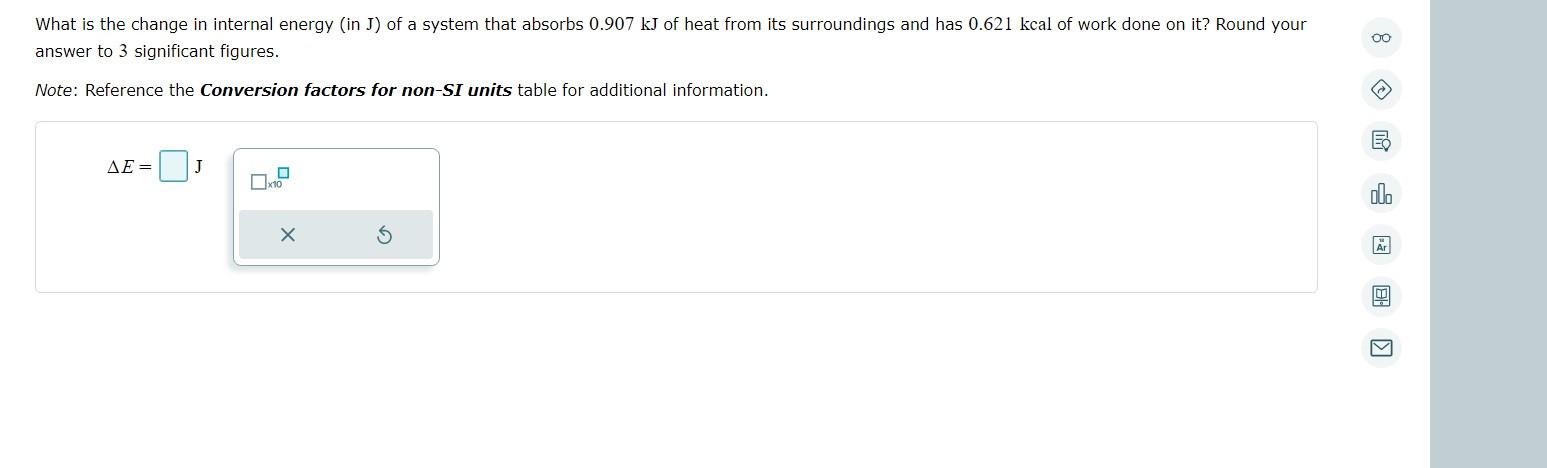
Solved What is the change in internal energy (in J) of a
1. Calculate the internal energy change for each of the following

SOLVED: 1.3) A system releases 125 kJ of heat while 104 KJ of work

Irrigation and Drainage Engineering 9783319056999, 3319056999

A system absorbs 50 kJ heat and does 20 kJ of work. What is the

Calculate the internal energy change for each of the following process

PDF) Theory & Problem of Heat Transfer

1. Calculate the internal energy change for each of the following