Solved IP A 35−g ice cube at 0.0∘C is added to 120 g of

Answer to Solved IP A 35−g ice cube at 0.0∘C is added to 120 g of

The specific heat capacity of liquid water is 4.18 kJ/g C, how would you calculate the quantity of energy required to heat 1.00 g of water from 26.5 C to 83.7 C?

Solved An ice cube with a mass of 53.0g at 0.0°C is added

Physics Textbook, PDF, Torque


How much energy is required to change a 35-g ice cube from ice at -15 deg C to steam at 120 deg C?

Solved How much energy is required to change a 35 g ice cube
How much energy is required to change a 35 g ice cube from ice at -15 degrees C to steam at 120 degrees C? - Quora

19-20) A 35-g ice cube at its melting point is dropped into an
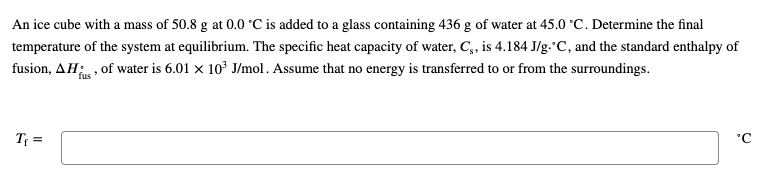
Solved An ice cube with a mass of 50.8 g50.8 g at 0.0 ∘C0.0