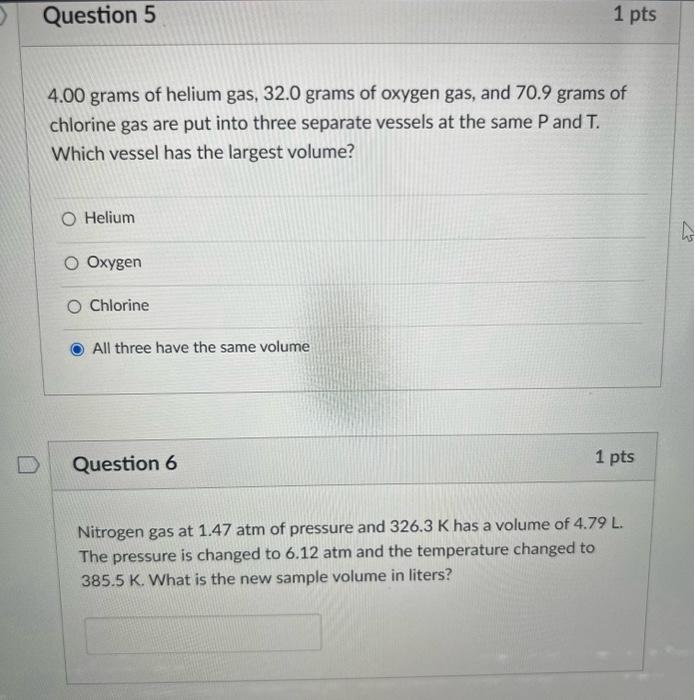
4. A container contains 32 g of O2 at a temperature TThe pressure
4. A container contains 32 g of O2 at a temperature TThe pressure of the gas is P. An identical containercontaining 4 g of H2 at a temperature 2T has apressure of(1) 8P(3) P(2) 4P(4) P18r cnstant
4- A container contains 32 g of O2 at a temperature TThe pressure of the gas is P- An identical containercontaining 4 g of H2 at a temperature 2T has apressure of-1- 8P-3- P-2- 4P-4- P18r-cnstant
A mixture of 40 g of oxygen and 40 g of helium has a total pressure of 0.9 atm. The partial pressure of oxygen is (1) 0.5 atm (2) 0.1 atm (3) 09 atm (4) 0.2 atm
Solved The pressure of a gas is initially 5.931 atmospheres.

A vessel contains 28 gm of N−2 and 32 gm of O2 temperature T = 1800 K and pressure 2 atm pressure it N2 dissociates 30 and O2 dissociates 50 temperature remains constant.

2.8 g of N2, 0.40 g of H2 and 6.4 g of O2 placed in a container of 1.0 L capacity 27°C. The total pressure in the container is : A) 6.15

A sealed container contains a mixture of oxygen and nitrogen gas.The ratio is .The ratio is
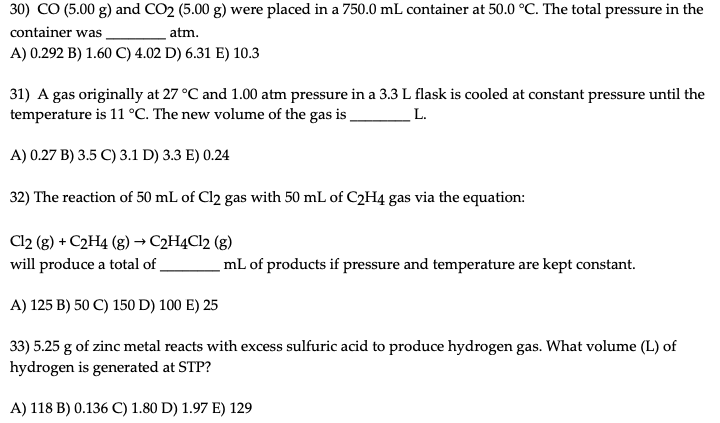
30) Co (5.00 g) and CO2 (5.00 g) were placed in a

toppr-doubts-media.s3.aws.com/images/1818262
What pressure is exerted by the mixture of 2.0g of hydrogen and 8.0 g of nitrogen at 273k in a 10l vessel? - Quora

Solved Information The pressure due to one component of a
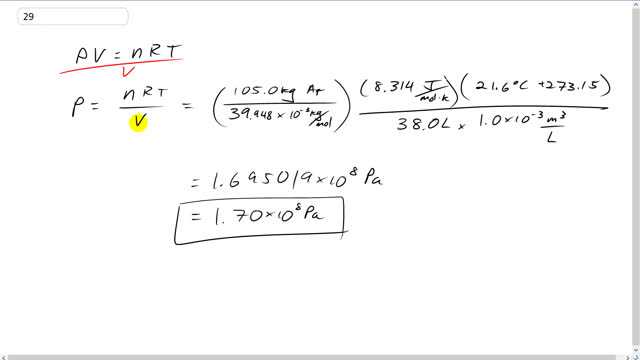
Giancoli 7th Edition, Chapter 13, Problem 29