32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
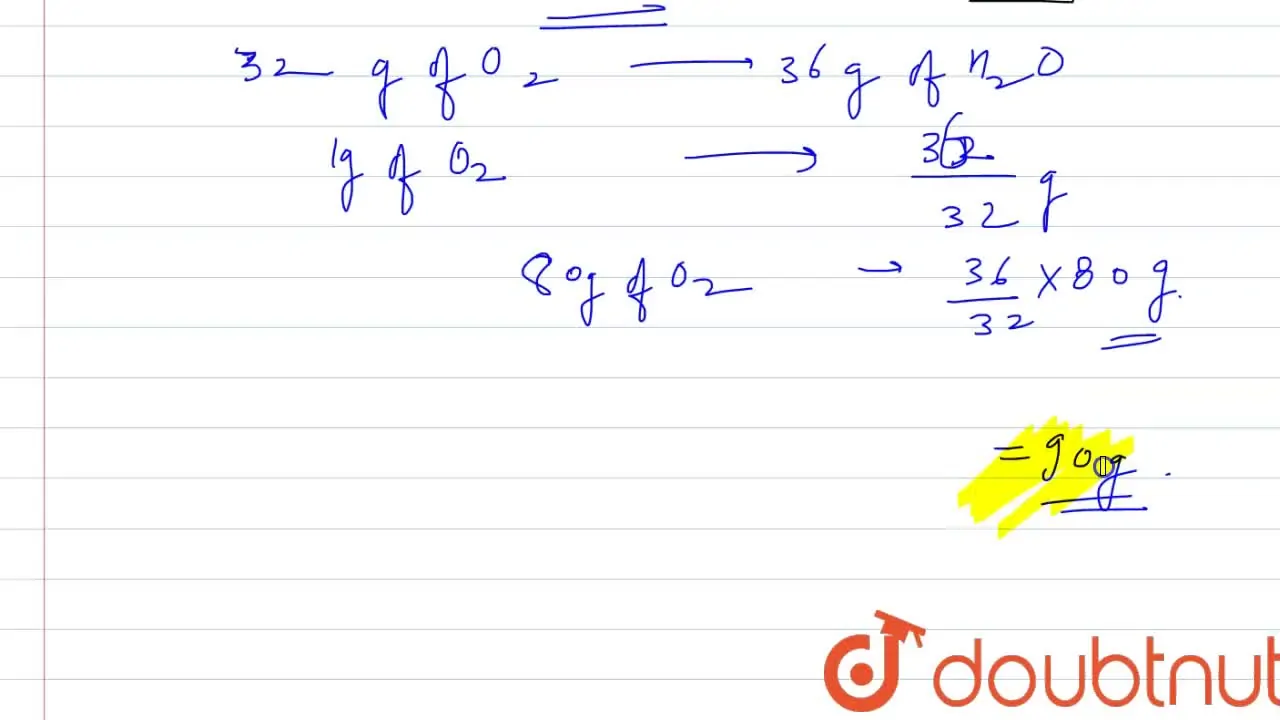
80 g of H(2) is reacted with 80 g of O(2) to form water. Find out the

Consider the following equation: 2 ClF3(g) + 2 NH3(g) → 1 N2
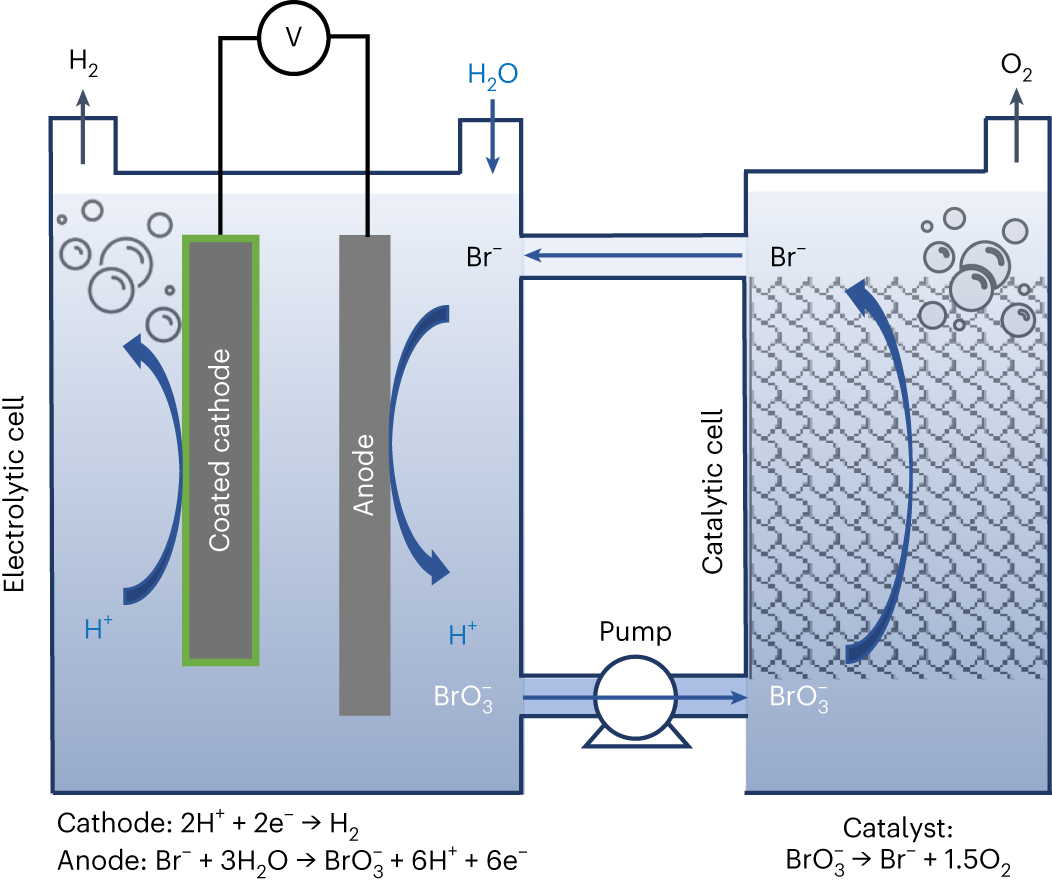
Electrochemical and chemical cycle for high-efficiency decoupled water splitting in a near-neutral electrolyte

ugures. i 64 of H, reacts with 32 g of Oz to yield water. Which is the limiting reactant? mass of water produced and the amount of excess reagent left. i) Explain
If 10 cm3 of each hydrogen and oxygen gases react to form water, what will the limiting reagent be? - Quora

526128650-Limiting-Reactants-and-the-Product-Formed.pdf

Kinetics of oxidation (Cl − /Cl2 and H2O/O2) and reduction kinetics

103 questions with answers in HYDRAZINE
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting reagent ii)Max amount of H2O formed iii) Amount of reactants which remains unreacted.

Spatiotemporal Decoupling of Water Electrolysis for Dual-Use Grid Energy Storage and Hydrogen Generation - ScienceDirect
Catalysts, Free Full-Text